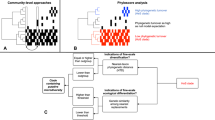
Abstract
Glacier-fed streams (GFS) feature among Earth’s most extreme aquatic ecosystems marked by pronounced oligotrophy and environmental fluctuations. Microorganisms mainly organize in biofilms within them, but how they cope with such conditions is unknown. Here, leveraging 156 metagenomes from the Vanishing Glaciers project obtained from sediment samples in GFS from 9 mountains ranges, we report thousands of metagenome-assembled genomes (MAGs) encompassing prokaryotes, algae, fungi and viruses, that shed light on biotic interactions within glacier-fed stream biofilms. A total of 2,855 bacterial MAGs were characterized by diverse strategies to exploit inorganic and organic energy sources, in part via functional redundancy and mixotrophy. We show that biofilms probably become more complex and switch from chemoautotrophy to heterotrophy as algal biomass increases in GFS owing to glacier shrinkage. Our MAG compendium sheds light on the success of microbial life in GFS and provides a resource for future research on a microbiome potentially impacted by climate change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All sequencing raw data and MAGs are deposited in NCBI under bioproject PRJNA781406. The MAGs annotations are deposited in Zenodo at https://doi.org/10.5281/zenodo.13890040 (ref. 97). Source data are provided with this paper.
Code availability
The code and data used in this study to create the figures are available in GitHub at https://github.com/michoug/VanishingGlaciersRcode (ref. 98). The code for binning is also available in GitHub at https://github.com/michoug/VanishingGlacierMAGs (ref. 99).
References
Milner, A. M. et al. Glacier shrinkage driving global changes in downstream systems. Proc. Natl Acad. Sci. USA 114, 9770–9778 (2017).
Cauvy-Fraunié, S. & Dangles, O. A global synthesis of biodiversity responses to glacier retreat. Nat. Ecol. Evol. 3, 1675–1685 (2019).
Wilkes, M. A. et al. Glacier retreat reorganizes river habitats leaving refugia for Alpine invertebrate biodiversity poorly protected. Nat. Ecol. Evol. 7, 841–851 (2023).
Wilhelm, L., Singer, G. A., Fasching, C., Battin, T. J. & Besemer, K. Microbial biodiversity in glacier-fed streams. ISME J. 7, 1651–1660 (2013).
Ezzat, L. et al. Global diversity and biogeography of the glacier-fed stream bacterial microbiome. Nature (in the press).
Battin, T. J., Besemer, K., Bengtsson, M. M., Romani, A. M. & Packmann, A. I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 14, 251–263 (2016).
Boix Canadell, M. et al. Regimes of primary production and their drivers in Alpine streams. Freshw. Biol. 66, 1449–1463 (2021).
Busi, S. B. et al. Genomic and metabolic adaptations of biofilms to ecological windows of opportunity in glacier-fed streams. Nat. Commun. 13, 2168 (2022).
Busi, S. B. et al. Cross-domain interactions confer stability to benthic biofilms in proglacial streams. Front. Microbiomes https://doi.org/10.3389/frmbi.2023.1280809 (2024).
Rott, E., Cantonati, M., Füreder, L. & Pfister, P. Benthic algae in high altitude streams of the Alps – a neglected component of the aquatic biota. Hydrobiologia 562, 195–216 (2006).
Peter, H., Michoud, G., Busi, S. B. & Battin, T. J. The role of phages for microdiverse bacterial communities in proglacial stream biofilms. Front. Microbiomes https://doi.org/10.3389/frmbi.2023.1279550 (2024).
Bourquin, M. et al. The microbiome of cryospheric ecosystems. Nat. Commun. 13, 3087 (2022).
Ezzat, L. et al. Benthic biofilms in glacier-fed streams from Scandinavia to the Himalayas host distinct bacterial communities compared with the streamwater. Appl. Environ. Microbiol. 88, e00421–e00422 (2022).
Ren, Z., Gao, H., Elser, J. J. & Zhao, Q. Microbial functional genes elucidate environmental drivers of biofilm metabolism in glacier-fed streams. Sci. Rep. 7, 12668 (2017).
Michoud, G. et al. Unexpected functional diversity of stream biofilms within and across proglacial floodplains despite close spatial proximity. Limnol. Oceanogr. 68, 2183–2194 (2023).
Michoud, G. et al. The dark side of the moon: first insights into the microbiome structure and function of one of the last glacier-fed streams in Africa. R. Soc. Open Sci. 10, 230329 (2023).
Busi, S. B. et al. Glacier-fed stream biofilms harbor diverse resistomes and biosynthetic gene clusters. Microbiol. Spectr. 11, e04069-22 (2023).
Kohler, T. J. et al. Global emergent responses of stream microbial metabolism to glacier shrinkage. Nat. Geosci. 17, 309–315 (2024).
Royo-Llonch, M. et al. Compendium of 530 metagenome-assembled bacterial and archaeal genomes from the polar Arctic Ocean. Nat. Microbiol. 6, 1561–1574 (2021).
Garner, R. E. et al. A genome catalogue of lake bacterial diversity and its drivers at continental scale. Nat. Microbiol. 8, 1920–1934 (2023).
Bowers, R. M. et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol. 35, 725–731 (2017).
Liu, Y. et al. A genome and gene catalog of glacier microbiomes. Nat. Biotechnol. 40, 1341–1348 (2022).
Paoli, L. et al. Biosynthetic potential of the global ocean microbiome. Nature 607, 111–118 (2022).
Tian, C. et al. Microbial community structure and metabolic potential at the initial stage of soil development of the glacial forefields in Svalbard. Microb. Ecol. 86, 933–946 (2023).
Boyd, E. S., Skidmore, M., Mitchell, A. C., Bakermans, C. & Peters, J. W. Methanogenesis in subglacial sediments. Environ. Microbiol. Rep. 2, 685–692 (2010).
Hotaling, S. et al. Microbial assemblages reflect environmental heterogeneity in alpine streams. Glob. Change Biol. 25, 2576–2590 (2019).
Tolotti, M. et al. Alpine headwaters emerging from glaciers and rock glaciers host different bacterial communities: ecological implications for the future. Sci. Total Environ. 717, 137101 (2020).
Fell, S. C. et al. Fungal decomposition of river organic matter accelerated by decreasing glacier cover. Nat. Clim. Change 11, 349–353 (2021).
Kohler, T. J. et al. Glacier shrinkage will accelerate downstream decomposition of organic matter and alters microbiome structure and function. Glob. Change Biol. 28, 3846–3859 (2022).
Niedrist, G. H. & Füreder, L. When the going gets tough, the tough get going: the enigma of survival strategies in harsh glacial stream environments. Freshw. Biol. 63, 1260–1272 (2018).
Longcore, J. E., Qin, S., Simmons, D. R. & James, T. Y. Quaeritorhiza haematococci is a new species of parasitic chytrid of the commercially grown alga, Haematococcus pluvialis. Mycologia 112, 606–615 (2020).
Nayfach, S. et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 39, 578–585 (2021).
Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (Princeton Univ. Press, 1968).
Székely, A. J., Berga, M. & Langenheder, S. Mechanisms determining the fate of dispersed bacterial communities in new environments. ISME J. 7, 61–71 (2013).
Tian, R. et al. Small and mighty: adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome 8, 51 (2020).
Alonso-Sáez, L. et al. Role for urea in nitrification by polar marine Archaea. Proc. Natl Acad. Sci. USA 109, 17989–17994 (2012).
Greening, C. & Grinter, R. Microbial oxidation of atmospheric trace gases. Nat. Rev. Microbiol. 20, 513–528 (2022).
Johnson, D. R., Goldschmidt, F., Lilja, E. E. & Ackermann, M. Metabolic specialization and the assembly of microbial communities. ISME J. 6, 1985–1991 (2012).
Wu, Z. et al. Single-cell measurements and modelling reveal substantial organic carbon acquisition by Prochlorococcus. Nat. Microbiol. 7, 2068–2077 (2022).
Olson, D. K., Yoshizawa, S., Boeuf, D., Iwasaki, W. & DeLong, E. F. Proteorhodopsin variability and distribution in the North Pacific Subtropical Gyre. ISME J. 12, 1047–1060 (2018).
Yurkov, V. V. & Beatty, J. T. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62, 695–724 (1998).
Tanabe, Y., Yamaguchi, H., Yoshida, M., Kai, A. & Okazaki, Y. Characterization of a bloom-associated alphaproteobacterial lineage, ‘Candidatus Phycosocius’: insights into freshwater algal-bacterial interactions. ISME Commun. 3, 20 (2023).
Ferrera, I., Sánchez, O., Kolářová, E., Koblížek, M. & Gasol, J. M. Light enhances the growth rates of natural populations of aerobic anoxygenic phototrophic bacteria. ISME J. 11, 2391–2393 (2017).
Reis-Mansur, M. C. P. P. et al. Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci. Rep. 9, 9554 (2019).
Williamson, R. J., Entwistle, N. S. & Collins, D. N. Meltwater temperature in streams draining Alpine glaciers. Sci. Total Environ. 658, 777–786 (2019).
Breitbart, M., Bonnain, C., Malki, K. & Sawaya, N. A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 3, 754–766 (2018).
Bäumgen, M., Dutschei, T. & Bornscheuer, U. T. Marine polysaccharides: occurrence, enzymatic degradation and utilization. ChemBioChem 22, 2247–2256 (2021).
Sichert, A. et al. Verrucomicrobia use hundreds of enzymes to digest the algal polysaccharide fucoidan. Nat. Microbiol. 5, 1026–1039 (2020).
Helbert, W. Marine polysaccharide sulfatases. Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00006 (2017).
Burke, C., Steinberg, P., Rusch, D., Kjelleberg, S. & Thomas, T. Bacterial community assembly based on functional genes rather than species. Proc. Natl Acad. Sci. USA 108, 14288–14293 (2011).
Rier, S. T., Shirvinski, J. M. & Kinek, K. C. In situ light and phosphorus manipulations reveal potential role of biofilm algae in enhancing enzyme‐mediated decomposition of organic matter in streams. Freshw. Biol. 59, 1039–1051 (2014).
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J. & Smith, A. G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93 (2005).
Amin, S. A. et al. Photolysis of iron–siderophore chelates promotes bacterial–algal mutualism. Proc. Natl Acad. Sci. USA 106, 17071–17076 (2009).
Kost, C., Patil, K. R., Friedman, J., Garcia, S. L. & Ralser, M. Metabolic exchanges are ubiquitous in natural microbial communities. Nat. Microbiol. 8, 2244–2252 (2023).
Seymour, J. R., Amin, S. A., Raina, J.-B. & Stocker, R. Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2, 17065 (2017).
Wadham, J. L., Bottrell, S., Tranter, M. & Raiswell, R. Stable isotope evidence for microbial sulphate reduction at the bed of a polythermal high Arctic glacier. Earth Planet. Sci. Lett. 219, 341–355 (2004).
Busi, S. B. et al. Optimised biomolecular extraction for metagenomic analysis of microbial biofilms from high-mountain streams. PeerJ 8, e9973 (2020).
Narayanasamy, S. et al. IMP: a pipeline for reproducible reference-independent integrated metagenomic and metatranscriptomic analyses. Genome Biol. 17, 260 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, D., Liu, C.-M., Luo, R., Sadakane, K. & Lam, T.-W. W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Hickl, O., Queirós, P., Wilmes, P., May, P. & Heintz-Buschart, A. binny: an automated binning algorithm to recover high-quality genomes from complex metagenomic datasets. Brief. Bioinform. 23, bbac431 (2022).
Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Wu, Y.-W. W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607 (2015).
Sieber, C. M. K. et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 3, 836–843 (2018).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://doi.org/10.48550/arXiv.1303.3997 (2013).
Shen, W., Le, S., Li, Y. & Hu, F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 11, e0163962 (2016).
Alneberg, J. et al. Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146 (2014).
Wang, Z., Huang, P., You, R., Sun, F. & Zhu, S. MetaBinner: a high-performance and stand-alone ensemble binning method to recover individual genomes from complex microbial communities. Genome Biol. 24, 1 (2023).
Chklovski, A., Parks, D. H., Woodcroft, B. J. & Tyson, G. W. CheckM2: a rapid, scalable and accurate tool for assessing microbial genome quality using machine learning. Nat. Methods 20, 1203–1212 (2023).
Vollmers, J., Wiegand, S., Lenk, F. & Kaster, A.-K. How clear is our current view on microbial dark matter? (Re-)assessing public MAG & SAG datasets with MDMcleaner. Nucleic Acids Res. 50, e76 (2022).
Olm, M. R., Brown, C. T., Brooks, B. & Banfield, J. F. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Huerta-Cepas, J. et al. EggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2019).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Aroney, S. T. N. et al. CoverM: read coverage calculator for metagenomics. Zenodo https://doi.org/10.5281/zenodo.10531253 (2024).
Chaumeil, P.-A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 36, 1925–1927 (2020).
Anantharaman, K. et al. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat. Commun. 7, 13219 (2016).
Karaoz, U. & Brodie, E. L. microTrait: a toolset for a trait-based representation of microbial genomes. Front. Bioinform. 2, 918853 (2022).
Stam, M. et al. SulfAtlas, the sulfatase database: state of the art and new developments. Nucleic Acids Res. 51, D647–D653 (2023).
Dai, C. et al. QSP: an open sequence database for quorum sensing related gene analysis with an automatic annotation pipeline. Water Res. 235, 119814 (2023).
Kurokawa, M. et al. Metagenomic thermometer. DNA Res. 30, dsad024 (2023).
Shaw, J. & Yu, Y. W. Fast and robust metagenomic sequence comparison through sparse chaining with skani. Nat. Methods 20, 1661–1665 (2023).
Rodriguez-R, L. M. et al. An ANI gap within bacterial species that advances the definitions of intra-species units. MBio 15, e0269623 (2024).
Pronk, L. J. U. & Medema, M. H. Whokaryote: distinguishing eukaryotic and prokaryotic contigs in metagenomes based on gene structure. Microb. Genom. 8, mgen000823 (2022).
Manni, M., Berkeley, M. R., Seppey, M., Simão, F. A. & Zdobnov, E. M. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 38, 4647–4654 (2021).
Tice, A. K. et al. PhyloFisher: a phylogenomic package for resolving eukaryotic relationships. PLoS Biol. 19, e3001365 (2021).
Neely, C. J., Hu, S. K., Alexander, H. & Tully, B. J. The high-throughput gene prediction of more than 1,700 eukaryote genomes using the software package EukMetaSanity. Preprint at bioRxiv https://doi.org/10.1101/2021.07.25.453296 (2021).
Kieft, K., Zhou, Z. & Anantharaman, K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome 8, 90 (2020).
Johansen, J. et al. Genome binning of viral entities from bulk metagenomics data. Nat. Commun. 13, 965 (2022).
Kieft, K., Adams, A., Salamzade, R., Kalan, L. & Anantharaman, K. vRhyme enables binning of viral genomes from metagenomes. Nucleic Acids Res. 50, e83 (2022).
Menzel, P., Ng, K. L. & Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 7, 11257 (2016).
Camargo, A. P. et al. Identification of mobile genetic elements with geNomad. Nat. Biotechnol. 42, 1303–1312 (2024).
Roux, S. et al. iPHoP: an integrated machine learning framework to maximize host prediction for metagenome-derived viruses of archaea and bacteria. PLoS Biol. 21, e3002083 (2023).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.r-project.org/ (R Foundation for Statistical Computing, 2021).
Patil, I. Visualizations with statistical details: the ‘ggstatsplot’ approach. J. Open Source Softw. 6, 3167 (2021).
Hackl, T., Ankenbrand, M. J. & van Adrichem, B. gggenomes: a grammar of graphics for comparative genomics. GitHub https://github.com/thackl/gggenomes (2024).
Michoud, G. et al. MAGs from glacier-fed streams. Zenodo https://doi.org/10.5281/zenodo.13890040 (2024).
Michoud, G. Vanishing Glaciers MAGs R Code. GitHub https://github.com/michoug/VanishingGlaciersRcode (2024).
Michoud, G., Bourquin, M. & Busi, S. B. Vanishing Glaciers MAGs pipeline. GitHub https://github.com/michoug/VanishingGlacierMAGs (2024).
Acknowledgements
The Vanishing Glaciers project is supported by The NOMIS Foundation (to T.J.B.) We thank A. McIntosh and L. Morris of New Zealand; J. Abermann and T. Juul-Pedersen of Greenland; O. Solomina and T. Kuderina Maratovna of Russia; V. Crespo-Pérez and P. Andino Guarderas of Ecuador; J. Yde and S. Leth Jørgensen of Norway; S. Sharma and P. Joshi of Nepal; N. Shaidyldaeva-Myktybekovna and R. Kenzhebaev of Kyrgyzstan; J. Nattabi Kigongo, R. Nalwanga and C. Masembe of Uganda; and M. Gonzlaléz and J. Luis Rodriguez of Chile for logistical support (see https://www.glacierstreams.ch for all institutions involved in the logistics of the expeditions). We particularly acknowledge the help of porters and guides in Nepal, Uganda and Kyrgyzstan; E. Oppliger for general laboratory support; and the Functional Genomics Centre Zurich for DNA sequencing. T.J.K. was also supported by the Charles University project PRIMUS/22/SCI/001. S.B.B. was supported by the Swiss National Science Foundation grant CRSII5_180241 to T.J.B.
Author information
Authors and Affiliations
Consortia
Contributions
G.M. was responsible for conceptualization, methodology, software, data curation, investigation, formal analysis, visualization and writing of the original draft. H.P. was responsible for conceptualization, methodology, data curation, investigation, formal analysis, visualization and writing of the original draft. S.B.B. and M.B. were responsible for the methodology, software, data curation, investigation and formal analysis. T.J.K. performed conceptualization and writing of the original draft. A.G., L.E. and the V.G.F.T. developed methodology and conducted investigations. T.J.B. was responsible for conceptualization, methodology, investigation, writing of the original draft, supervision, project administration and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Katie Sipes, David Walsh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
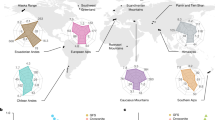
World map depicting the mountain ranges where the Vanishing Glaciers Project sampled glacier-fed streams studied in the present paper.
Extended Data Fig. 2 Maximum likelihood phylogenetic tree of the archaeal MAGs.
The colors of the branches correspond to the taxonomical affiliation of the MAGs at the class level. Then, the presence of similar MAGs (ANI% > 95%) in another dataset (GFS rocks from New Zealand and Caucasus8). The gradient colors from blue to yellow show the normalized abundance of the different MAGs.
Extended Data Fig. 3 Comparative genomic organization of the mcrABDG cluster across the GFS_11005 (a) and GFS_3223 MAGs (b) and their closest relatives.
Extended Data Fig. 4 Eukaryotic MAGs sourced from glacier-fed streams sampled by the Vanishing Glaciers project.
Maximum likelihood phylogenetic tree of the eukaryotic MAGs and reference genomes. The colors of the branches correspond to the taxonomic affiliation of MAGs at phylum level. The black color represents other branches of the eukaryotic tree of life. MAGs are indicated by the red dots. The gradient colors from blue to yellow show the normalized abundance of the different MAGs.
Extended Data Fig. 5
Differences in numbers (a,b) and relative abundance (c,d) of MAGs classified as specialists or generalists based on the levins index.
Extended Data Fig. 6
Barplot indicative of the predicted trophic state of the MAGs present in the different functional clusters either characterized as specialists (blue) or generalists (red).
Extended Data Fig. 7 Differences in estimated genome length between the pMAGs classified as specialists or generalists.
Mann-Whitney U Test showed a statistical difference between generalist and specialist genome length (U = 3.26 × 105, p = 1.42 × 10−9). Red circles show the medians while the box limits denote the 25th and 75th percentiles. The solid lines extend 1.5 times the interquartile range from these percentiles. The polygons represent density estimates of the data. The plot was made with ggstatsplot95.
Extended Data Fig. 8 Differences in genome length between the pMAGs present in the different functional clusters.
Red circles show the medians while the box limits denote the 25th and 75th percentiles. The solid lines extend 1.5 times the interquartile range from these percentiles. The polygons represent density estimates of the data. The plot was made with ggstatsplot95.
Extended Data Fig. 9
Presence of genes linked to photrophy per functional cluster.
Supplementary information
Supplementary Tables 1–6
Supplementary Table 1 General physicochemical characteristics of the different samples selected in this study. Table 2 General features and statistics of the metagenomes in glacier-fed streams. Table 3 General features and characteristics of the prokaryotic MAGs in glacier-fed streams. Table 4 General features and characteristics of the eukaryotic MAGs in glacier-fed streams. Table 5 Carotenoid genes coverage and originating MAG phylogeny. Table 6 List of genes potentially involved in the relationship between bacteria and algae in glacier-fed streams.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michoud, G., Peter, H., Busi, S.B. et al. Mapping the metagenomic diversity of the multi-kingdom glacier-fed stream microbiome. Nat Microbiol 10, 217–230 (2025). https://doi.org/10.1038/s41564-024-01874-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01874-9